Combined MRI and PET (Multimodality Imaging) in anesthetized Mice and Rats has become routine in the MRRC, as the studies exemplified below indicate. In some cases, specialized MRI sequences have been developed to complement the PET coregistration requirements. Investigators interested in their own Multimodality experiments should contact the MRRC staff. The experiments illustrated here were conducted with the expert guidance of
Dr. Min-Hui Cui Dr. Linda Jelicks.
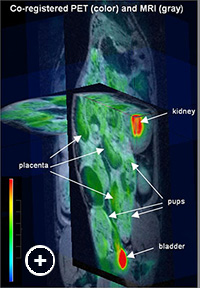
Fig. 1: 3D presentation of co-registered MRI (in grayscale) and FDG-PET (in color) images of a pregnant rat. Color bar shows greatest uptake is red (kidney, bladder). Fetal Multimodality Imaging
The field of study of maternal-fetal exchange has been a significant and well researched topic due to the importance of understanding how nutrients as well as toxins travel between mother and fetus through the placenta. Maternal nutrition and the environment of the fetus affect fetal phenotype during childhood and adulthood. Studies have shown that maternal diabetes and therefore states of hyperglycemia cause increased insulin production in the fetus leading to large fetal size. These babies are more likely to develop diabetes and metabolic syndrome.
Poor maternal nutrition has been shown to lead to growth restriction and small weight babies. Furthermore, these babies are then also predisposed to developing diabetes and metabolic disease. The exchange of nutrients in the placenta is vital and the mechanisms by which fetal life can change phenotype in later life have not been elucidated. In vitro methods of studying maternal-fetal transfer are limited because these are not able to detect how the fetus is affected physiologically and therefore we are interested in studying this condition in vivo.
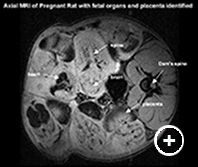
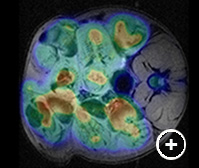
Fig. 2: Axial MRI (left) of a pregnant rat (Dam) showing several fetal-placental units with organ identification. Co-registration with the FDG-PET image (right) permits quantitation of glucose uptake in specific regions of interest.The laboratory of Dr. Francine Einstein previously used 18F-FDG Micro Positron Emission Tomography (microPET) to study and quantify in vivo tissue glucose uptake to evaluate the effect of chronic maternal diets and acute lipid load on fetal-placental glucose uptake. They have shown that there is an increase in glucose uptake in the placental fetal units of calorie-restricted as well as high fed rats; however, with microPET alone, they were unable to differentiate fetus from placenta in the fetal-placental units. MRI is a safe and non invasive technique that can be paired with microPET scanning to allow better visualization of fetal and placental glucose uptake. The 3D isotropic T1 weighted MRI scan provides an accurate anatomical map that can be co-registered with the micro PET data. By combining MRI with the micro PET scan we can delineate many fetal structures and the placenta and quantify glucose uptake in both maternal and fetal organs.
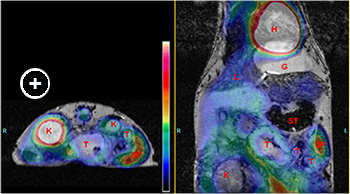
Fig. 2: Left: cross-section at the level of the large tumor). Right: coronal image, anterior to left panel) co-registered MRI (in grayscale) and FDG-PET (in color) images of the mouse showing FDG uptake only at the periphery of the large tumor. The color bar indicates that the greatest FDG uptake (white) in heart and kidney. 
Fig.1: Left: MRI image of a Men1 KO mouse showing gallbladder (G), kidney (K), liver (L), stomach (ST), spleen (SP) and pancreatic tumors (T). Right: In vivo 1H MRS spectrum on pancreatic tumor of Men1 KO mouse showing total choline (tCho) peak at 3. 2 ppm.Multimodaity Imaging of PancreaticTumors
The laboratory of Dr. Steven Libutti developed animal models of pancreatic neuroendocrine tumors allowing for informative preclinical studies of targeted antitumor agents. They previously used 18F-FDG PET/CT to evaluate the effect of pasireotide on tumor growth in the mice (1). While CT provides excellent high-resolution images of skeletal structures there is little contrast between soft tissues confounding. MRI provides excellent soft tissue contrast and provides an anatomic template for co-registration with 18F-FDG PET that permits evaluation of glucose uptake in tumors. In addition, MRS has identified choline signatures of the tumor, and identified tumor masses in other tissues in this animal model, which were not previously known to exist.
- Pasireotide (SOM230) is effective for the treatment of pancreatic neuroendocrine tumors (PNETs) in a multiple endocrine neoplasia type 1 (MEN1) conditional knockout mouse model.
Quinn TJ, Yuan Z, Adem A, Geha R, Vrikshajanani C, Koba W, Fine E, Hughes DT, Schmid HA, Libutti SK. Surgery. 2012 Dec;152(6):1068-77.
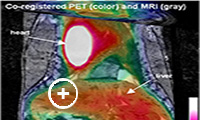
Fig. 1: Co-registered MRI (in grayscale) and FDG-PET (in color) images of a mouse showing FDG uptake in regions of the intestinal tract. A segment of the colon with enhanced uptake is outlined in semi-transparent purple. The color bar indicates that the greatest uptake is white (heart and bladder).Multimodality Imaging of Intestinal Tumors
Data from the laboratory of Dr. Leonard Augenlicht suggests that the inheritance of a mutant Apc allele that increases probability of intestinal tumor development in the mouse, or the feeding of a purified western-style diet that mimics intake of major nutritional risk factors for human colon cancer, shifts the intestinal mucosa towards a more glycolytic phenotype. This takes place well before tumors arise in either the genetic or the dietary mouse model. Exploratory experiments were performed to determine if 18F-FDG PET imaging can be used to detect this shift towards glycolytic metabolism in the colon and intestine of mice. While growing tumors produce a significant PET image and readily detected, the signal from the intestine is generally low. MRI was used as a means to map position of anatomical structures, including the intestine, providing a template for quantitative analysis of the PET signal in regions of the intestine.